
Kbr molar mass full#
I'm going to keep this as a full decimal, can not round until a final answer, so I could get a more accurate answer. But we have so concentration is 0.3 55 bowlers in the volumes is 0.225 liters. We have volume, and we can rearrange this equation to solve formals and plug in.

So now that we have volume and concentration, we can solve for moles using the formula, concentration equals most over volume and we have concentration. So 225 milliliters is equal to 0.225 leaders because there is 1000 milliliters in a leader. So we were given the volume in milliliters, and I went ahead to convert that to leaders as the formulas we use uses leaders instead of the leaders. The question is asking us to sold for the mass of the Soul Ute when given a volume of 0.225 leaders and a concentration of 0.35 molars. This gives us 8.13 times 10 to the negative three. 0.1230 over the leaders over a solution which is 1.1 1.60 meter solving. We have the most of our soldier, which is K p R.
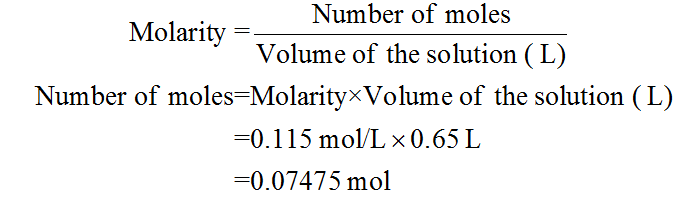
All right now we have everything we need. We have one mole of cave life, and it simplifies to 0.130 most of K B R. All right, so now that we've found the more mass we convert our Graham two more for one 119 grams of Dave. Except I'm going around that 219 Grant Trimble. Quite 10 for K and B always 79.9, we have 79.9 come together.
Kbr molar mass plus#
All right, that looks like 39.98 So 39.98 plus b r, which is 75. So what is the more massive Katie are? You see that the moves of K and let's see right here. So in order to do that, we need to find our molar mass of K B. So we have the leaders of our collusion, and we currently have our value, which is Katie our Ingrams. So what is the definition of polarity? Well, polarity is equal to Big M is equal to the most of our value over the leaders of our solution. Ah, 1.6 oh, leaders of solution containing 1.55 grams of results a B R. a pollution of 1 gram of benzene in a certain amount of water converts to N A/78.11≈ 7.It's a phrase question were asked to calculate the polarity. Using the above calculator you could find that e.g. Or 1 mole of a substance will contain Avogadro's number of that substance. The term " mole" is defined in that one mole of a substance with a molecular (or atomic) mass of one (1), will have a mass of 1 gram. It is defined to be 1/12 of the mass of one atom of carbon-12 and in older works is also abbreviated as "amu".Īlso, important in this field is Avogadro's number (N A) or Avogadro's constant (6.0221 x 10 23). In related terms, another unit of mass often used is Dalton (Da) or unified atomic mass unit (u) when describing atomic masses and molecular masses. Molecular mass or molar mass are used in stoichiometry calculations in chemistry. This Calculator has been tested on Internet Explorer version 6 only,įirefox might not show all fields correctly. For question or remarks please contact us. !!! Lenntech BV cannot be held responsible for errors in the calculation, Make sure you enter the molecule of crystallization at last (e.g. The calculator handles at most two different bracket levels. The molecular mass calculator will recognize the entered formula's, which are included in the list of organic compounds.
Kbr molar mass series#
Or you can choose by one of the next two option-lists, which contains a series of common organic compounds (including their chemical formula) and all the elements. This online calculator you can use for computing the average molecular weight (MW) of molecules by entering the chemical formulas (for example C3H4OH(COOH)3 ). Molecular Weight Calculator Molecular Weight Calculator


 0 kommentar(er)
0 kommentar(er)
